A trial looking at MEDI4736, savolitinib and tremelimumab for advanced kidney cancer (CALYPSO)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is for people with a type of kidney cancer called renal cell cancer that has grown outside the kidney or spread to another part of the body.
More about this trial
Renal cell cancer is the most common type of kidney cancer in adults. There are different types including:
- papillary kidney cancer
- clear cell kidney cancer
- sarcomatoid kidney cancer
Renal cell cancer can sometimes grow outside the kidney or spread to another part of the body. This is advanced kidney cancer.
Treatment for advanced kidney cancer is usually a biological therapy (or targeted treatment). In this trial doctors are looking at different biological therapies:
- MEDI4736
- savolitinib
- tremelimumab
MEDI4736 and tremelimumab are monoclonal antibodies. They seek out cancer cells by looking for particular proteins and attaching to them.
Researchers think that MEDI4736 and tremelimumab can help your  to attack the cancer and stop it from growing.
to attack the cancer and stop it from growing.
Savolitinib works by blocking a protein called MET. This protein sends signals to cells telling them to divide and grow. Blocking MET may stop cancer cells from growing.
In this trial, people with papillary kidney cancer have MEDI4736 and savolitinib. And people with clear cell and sarcomatoid kidney cancer have 1 of the following:
- MEDI4736
- savolitinib
- MEDI4736 and savolitinib
- MEDI4736 and tremelimumab
The main aims of this trial are to:
- find out how well the treatments work
- learn more about the side effects
- find out how cancer develops and who benefits from having MEDI4736, savolitinib and tremelimumab (translational research)
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
You may be able to join this trial if all of the following apply.
- You have a type of renal cell cancer called clear cell, papillary or sarcomatoid that has grown outside the kidney or spread to another part of the body
- Your cancer has grown after having had drugs that block a protein called
VEGF  such as cabozantinib, pazopanib, sorafenib and sunitinib – if you have clear cell or sarcomatoid renal cell cancer
such as cabozantinib, pazopanib, sorafenib and sunitinib – if you have clear cell or sarcomatoid renal cell cancer - If you have papillary renal cell cancer, you haven’t yet had drugs that block the VEGF protein (such as bevacizumab, pazopanib, sorafenib and sunitinib), or you have had it but it stopped working (treatment
refractory  )
) - You have at least 1 area of cancer that can be seen on a CT or MRI scan and measures at least 20 mm (or 10 mm if you had a type of CT scan called a spiral CT) and it hasn’t been treated with
radiotherapy  . The area of cancer mustn’t be in the bones or the fluid around your tummy (abdomen), lungs or heart
. The area of cancer mustn’t be in the bones or the fluid around your tummy (abdomen), lungs or heart - You are willing to have a sample of your cancer taken (a
biopsy  ) if there is no suitable sample available
) if there is no suitable sample available - You have satisfactory blood tests results
- You are able to swallow and absorb capsules
- You have a satisfactory heart rate
- You are well enough to carry out your normal activities, apart from heavy physical work (performance status of 0 or 1)
- You are at least 18 years old
- You are willing to use reliable contraception during treatment and for 90 days after the final dose of MEDI4736 and savolitinib, if there is any possibility you or your partner could become pregnant. If you have savolitinib alone, you must use reliable contraception for 30 days after treatment
You cannot join this trial if any of these apply.
Cancer related
- Your cancer has spread to your brain and you need to have treatment such as surgery, radiotherapy or steroids
- Your cancer has spread to the tissues (membranes) surrounding your brain (leptomeningeal disease)
- You have had treatment with cancer drugs that affect certain proteins such as PD-L1 (nivolumab), MET (cabozantinib) and CTLA (ipilimumab)
- You have had chemotherapy,
immunotherapy  , treatment with hormones (endocrine therapy) or a
, treatment with hormones (endocrine therapy) or a tumour embolisation  in the past 2 weeks
in the past 2 weeks - You have had radiotherapy or the drugs interferon and interleukin in the past 4 weeks
- You have moderate side effects from previous cancer treatment apart from hearing loss or numbness and tingling in your hands and feet (peripheral neuropathy)
- You have had severe side effects to
immunotherapy  treatment
treatment - You have had another cancer in the past 3 years apart from cancers that have not spread (
carcinoma in situ  ) of the cervix and prostate, non melanoma skin cancer or any other cancer that has been successfully treated and there is no signs of it coming back
) of the cervix and prostate, non melanoma skin cancer or any other cancer that has been successfully treated and there is no signs of it coming back
Medical conditions
- You take warfarin
- You have taken drugs that damp down your immune system (immunosuppressants) such as steroids in the past 3 weeks, unless it was a very small amount or an inhaler
- You have taken drugs that affect certain substances called CYP enzymes in the past 2 weeks (or 3 weeks for St John’s Wort)
- You have had a
stem cell transplant  using cells from a donor (allogeneic) or an organ transplant
using cells from a donor (allogeneic) or an organ transplant - You have had an
autoimmune disease  that needed treatment in the past 2 years, unless it was treatment to replace something the body makes such as thyroxin, vitiligo, Grave’s disease or psoriasis that hasn’t needed treatment that reached your whole body (systemic treatment)
that needed treatment in the past 2 years, unless it was treatment to replace something the body makes such as thyroxin, vitiligo, Grave’s disease or psoriasis that hasn’t needed treatment that reached your whole body (systemic treatment) - You have had a major surgery in the past 4 weeks, a small operation in the past week or your doctor thinks that you might need a large operation during this trial
- You have a
blood clot  and you are taking
and you are taking heparin  to treat this. You may be able to take part if you take a low dose (LMWH)
to treat this. You may be able to take part if you take a low dose (LMWH) - You have lung problems such as infection (pneumonia) or inflammation of the lung tissue (
pneumonitis  )
) - You have heart problems such as congestive heart failure that is causing symptoms, high blood pressure that is not controlled by medication, angina that is not controlled or you have had a heart attack in the past 3 months
- You have problems with your
digestive system  such as colitis, Crohn’s disease, a
such as colitis, Crohn’s disease, a peptic ulcer  or
or gastritis 
- You have type 1
diabetes 
- You have hepatitis B or hepatitis C
- You have HIV
- You have or have had tuberculosis (TB)
- You have liver problems such as cirrhosis, fits (seizures) or you have an obstruction in a large vein called superior vena cava
- You have another medical condition or mental health problem that the trial team think could affect you taking part
Other
- You are sensitive to MEDI4736, tremelimumab, savolitinib or anything they contain
- You have had a live
vaccination  in the past 30 days
in the past 30 days - You are pregnant or breastfeeding
Trial design
This is an international phase 2 trial. Researchers need about 195 people to take part worldwide and hope that around 110 people from the UK will take part.
This trial has 2 groups (or cohorts).
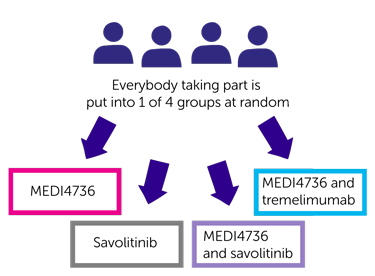
The first cohort is for people with clear cell or sarcomatoid kidney cancer. Researchers need about 156 people to join this group. The people taking part are put into 1 of the following treatment groups by computer:
- MEDI4736
- savolitinib
- MEDI4736 and savolitinib
- MEDI4736 and tremelimumab
Neither you nor your doctor will be able to decide which group you are.
The second cohort is for people with papillary kidney cancer. Researchers hope that up to 39 people will join this group. This group have MEDI4736 and savolitinib.
MEDI4736
You have MEDI4736 as a drip into a vein every 4 weeks.
You have it for as long as it works and the side effects aren’t too bad. You are likely to have it for up to 5 months (5 treatment cycles).
Savolitinib
Savolitinib comes as capsules. You take them once a day and for as long as it works. Doctors think you are likely to take savolitinib for up to 5 months.
MEDI4736 and savolitinib
First you take savolitinib capsules for 4 weeks. Then you have MEDI4736 and savolitinib together.
You have savolitinib capsules once, every day. And MEDI4736 as a drip into your vein, every 4 weeks. This continues for as long as the treatment is helping you and the side effects aren’t too bad.
MEDI4736 and tremelimumab
You have MEDI4736 and tremelimumab as a drip into your vein. You have both drugs every 4 weeks, for 16 weeks (about 4 months).
Then you have a lower dose of MEDI4736 every 4 weeks. You continue to have a lower dose of MEDI4736 for as long as it works.
Blood samples
You have some extra blood tests as part of this trial. Researchers want to find out more about how cancer develops and who would benefit most from having treatment with MEDI4736, savolitinib and tremelimumab.
You have the extra blood samples before the start of treatment and then:
- 2 weeks after the first dose of treatment (if you have clear cell or sarcomatoid kidney cancer)
- every 4 weeks for 12 weeks
- every time you have a CT or MRI scan
- at the end of your treatment
Tissue samples
The trial team will ask to use a tissue sample of your cancer taken either when you were diagnosed or during treatments. But if there isn’t a suitable sample, you might need to have a new sample taken (a biopsy) before the start of treatment.
The research team might also ask you to have extra biopsies. You have these:
- between 9 to 12 weeks after the start of treatment
- at the end of treatment
You don’t have to agree to have the extra biopsies if you don’t want to. You can still take part in this trial.
Hospital visits
You see a doctor and have some tests before taking part. These tests include:
After you start treatment, you see a doctor and have a physical examination every week if you have savolitinib or MEDI4736 and savolitinib. Or every 2 weeks if you have MEDI4736 and tremelimumab or MEDI4736. This continues for 8 weeks.
After 8 weeks everyone sees the trial doctor every 4 weeks.
You have a CT or MRI scan:
- 4 weeks after the start of treatment
- then every 8 weeks
This continues for as long as your cancer stays the same and does not get worse. If your cancer gets worse you stop having treatment.
When you finish treatment, you see the trial team and have some blood tests. You also have a CT or MRI scan. After this, you see them again after 3 months (or 1 month if you had savolitinib).
After 3 months the trial team will phone you every year to see how you are. This is for 2 years.
Side effects
This is the first time MEDI4736 will be given with savolitinib and tremelimumab to help people with kidney cancer. So there might be side effects we don’t know about yet.
The trial team monitor you during the time you have treatment and you have a phone number to call them if you are worried about anything. The team will tell you about all the possible side effects before you start the trial.
The most common side effect of MEDI4736 is tiredness (fatigue).
The most common side effects of savolitinib are:
- feeling or being sick
- tiredness (fatigue)
- fluid build up (or fluid retention)
The most common side effects of tremelimumab are:
- diarrhoea
- skin rash
- tiredness (fatigue)
- feeling or being sick
- loss of appetite
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Thomas Powles
Supported by
Astra Zeneca
Queen Mary University of London
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



