A trial of CD19 T cells for diffuse large B cell lymphoma (COBALT)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at the safety of CD19 T cells as a treatment for people with diffuse large B cell lymphoma before a donor stem cell transplant.
More about this trial
Chemotherapy with rituximab is  for diffuse large B cell lymphoma (DLBCL). Radiotherapy might also be a part of the treatment.
for diffuse large B cell lymphoma (DLBCL). Radiotherapy might also be a part of the treatment.
This works well but the lymphoma might come back and need further treatment. This is called salvage treatment. After salvage treatment some people might have a transplant using their own stem cells. This is an  .
.
But for some people the salvage treatment might not work well enough. For others their lymphoma might come back after the autologous stem cell transplant. Researchers are looking for new ways to treat these people.
This trial is looking at CD19 T cell treatment, also called CAR T-cell therapy. It is a type of adoptive cell transfer.
The trial team will use a type of white blood cell called lymphocytes. Lymphocytes are part of the immune system that helps protect the body against disease. The team takes some of your lymphocytes and changes them in the laboratory.
Your lymphocytes will be grown alongside a virus called lentivirus. The lentivirus has been changed so it can’t grow and cause an infection. But it has a gene that can recognise a certain protein called CD19. Some lymphoma cells have a high number of CD19 protein on their surface.
The researchers hope this gene will be passed on to your lymphocytes. So when you have these changed lymphocytes back they recognise the CD19 protein. And attack and kill the lymphoma cells. They hope this might also increase the chances of you being able to have a donor stem cell transplant ( ).
).
This is the first time this type of treatment has been used for people with DLBCL. So the researchers need to find if this type of treatment is safe for people with DLBCL before doing further trials. They also want to find:
- how well this treatment works for people with DLBCL
- the best dose to give
- if CD19 T cell treatment increases the possibility of having a donor stem cell transplant
Please note you might not get any benefit from taking part in this trial but the information gained could help people with DLBCL in the future.
Who can enter
The following bullet points list the entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
You may be able to join this trial if all of the following apply:
- you have diffuse large B cell lymphoma (DLBCL) that has the CD19 protein
- your lymphoma continued to get worse during, or came back after salvage treatment with chemotherapy or your lymphoma came back after an autologous stem cell transplant followed by more chemotherapy
- you are able to have a stem cell transplant from a donor (allogeneic stem cell transplant)
- you might need help but can mostly care for yourself (Karnofsky performance status 60 and above)
- you are willing to use reliable contraception during treatment and for a year afterwards
- you are between 16 and 65 years old
You cannot join this trial if any of these apply:
- you have lymphoma that has spread to your brain or spinal cord
- you have already had a donor transplant (allogeneic transplant)
- you have high blood pressure that isn’t controlled by medication
- you have heart problems such as ischemic heart disease, a heart trace (
ECG  ) that isn’t normal or problems with the rhythm of your heart
) that isn’t normal or problems with the rhythm of your heart - you have had a stroke in the past 3 months
- you have an
autoimmune disease  that needs medication that can affect how your immune system works
that needs medication that can affect how your immune system works - you have HIV, hepatitis B or hepatitis C
- you are taking more than 10mg per day of steroids
- you have had rituximab in the past 2 months
- you aren’t able to have any of the following chemotherapy drugs ifosfamide, epirubicin, etoposide, fludarabine and cyclophosphamide
- you are allergic to albumin or dimethyl sulfoxide (DMSO)
- you are pregnant or breastfeeding
Trial design
This is a phase 1 trial. The researchers need 12 people to join.
Everyone has treatment with CD19 T cells before been considered to have a donor stem cell transplant.
The first few people have a low dose of CD19 T cells. The next have a higher dose if the first few people didn’t have any serious side effects. And so on until the best dose of CD19 T cells is found. This is called a dose escalation study.
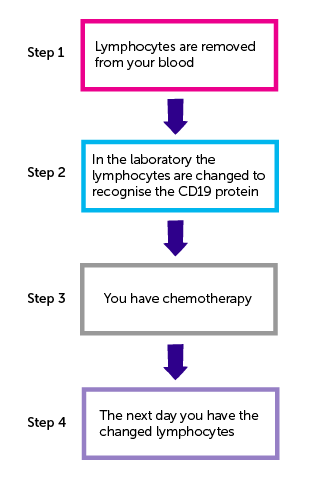
There are 4 steps to developing your CD19 T cell treatment.
Step 1
You have white blood cells called lymphocytes removed from your blood. This is called leukapheresis.
You lie on a bed or reclining chair with a tube into a vein in each arm. One tube removes blood and passes it into a machine that removes white blood cells including the lymphocytes. The rest of your blood cells and normal blood fluid (plasma) go back into your body through the tube in your other arm. You might have this done through your  if you have one in place.
if you have one in place.
Step 2
The lymphocytes are taken to the laboratory. In the laboratory your lymphocytes are grown alongside the lentivirus. Your lymphocytes pick up the gene that recognises the CD19 protein.
Step 3
You have 1 treatment of chemotherapy. This can include the following chemotherapy drugs:
The week before you have the changed lymphocytes you have
- cyclophosphamide on day 1 and day 2
- fludarabine on the remaining 5 days
This helps the changed lymphocytes to survive and grow.
You have the chemotherapy as a drip into a vein. Or through a central line if you have one in place.
Step 4
You have the changed lymphocytes the day after completing chemotherapy. You have the lymphocytes as a drip into a vein or through a central line.
Blood samples
You have blood tests:
- on the day you have the CD19 T cells
- then afterwards on days 3, 6, 8, 10, 13 and 20
- at 1 month
People who don’t have a transplant have more blood tests at:
- 2 months
- 3 months
- 6 months
- 9 months
- 12 months
Researchers use these blood samples to find out how well the changed lymphocytes survive and grow in your body and how well they work. They will also look for substances that show the lymphocytes are active.
Please note
Growing and changing the lymphocyte cells in the lab might not always work. Researchers think this could happen in 1 out of every 10 people.
Your doctor will talk to you about this and other treatments available if this happens to you.
Hospital visits
You see the doctor to have some tests before taking part. These include:
- a physical examination
- blood tests
- urine test
- heart trace (
ECG  )
) - PET-CT scan
You have your lymphocytes collected at the hospital. This takes between 2 and 4 hours. Afterwards you remain in the chair or bed for 20 minutes. You can then go home if you are feeling all right.
You have the chemotherapy before the CD 19 T cell treatment in the usual way.
You have the following tests before having the changed lymphocytes:
- a physical examination
- a test to check the level of oxygen in your blood (oxygen saturation)
- blood tests
- heart trace (if needed)
You have the lymphocytes at the hospital. It can take up to 20 minutes to give them. The team will monitor you closely while having the lymphocytes and for 4 hours afterwards.
After this you stay as part of the UCLH Ambulatory Care service for up to 10 days. This is so you can be monitored closely and admitted into hospital if necessary. People who are well enough can stay in a local hotel with a companion if they so desire. If you aren’t well enough you will stay in hospital.
At day 10 you see the doctor and have a heart trace done.
After leaving ambulatory care you see the doctor
- every week for a month
- then at 2 months
- 3 months
- 6 months
- 9 months
- 12 months
At these visits you will have a physical examination and blood tests.
You have a PET-CT scan 1 month after having the CD19 T cell treatment. This is to see how well the treatment worked and whether you are able to have the transplant.
You have another PET-CT scan at the 12 month visit if you don’t have the transplant.
After your CD19 T cell treatment you see the doctor every year for 3 years.
Side effects
CD19 T cell treatment is new. There might be side effects we don’t know about yet. You will be closely monitored for any side effects during the treatment and for 10 days afterwards.
The changed lymphocytes might cause allergic reactions such as a high temperature or a rash. But the team will give you other medication to try to stop this happening.
The lymphocytes might also target and kill some normal blood cells that help you fight infection. You may have drugs called immunoglobulins to help fight infections if this happens.
Your doctor will talk to about the side effects of CD19 T cell treatment before you agree to take part.
We have information about the side effects of
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Karl Peggs
Supported by
University College London (UCL)
Bloodwise
Cancer Research UK & UCL Cancer Trials Centre (UCL CTC)
NIHR Clinical Research Network: Cancer
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



