A trial looking at chemotherapy with or without bevacizumab for advanced ovarian cancer (ICON8B)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at chemotherapy with or without bevacizumab for advanced ovarian cancer, fallopian tube cancer or primary peritoneal carcinoma. These cancers are all treated in the same way, so when we use the term ovarian cancer in this summary, we are referring to all 3. This trial is supported by Cancer Research UK.
More about this trial
Doctors usually treat advanced ovarian cancer with surgery and chemotherapy. The most common chemotherapy drugs doctors give are carboplatin and paclitaxel. They can also use a biological therapy drug called bevacizumab.
You usually have chemotherapy once every 3 weeks. Each 3 week period is a cycle of treatment. You usually have 6 cycles all together. This is known as the 
Some women taking part have surgery first, followed by 6 cycles of chemotherapy. And some women have 3 cycles of chemotherapy first, then they have surgery followed by 3 more cycles of chemotherapy.
The aims of the trial are to see:
- if weekly chemotherapy with bevacizumab is better than 3 weekly chemotherapy with bevacizumab for women with advanced ovarian cancer
- which treatment causes more or fewer side effects
- which treatment offers better quality of life
- whether it is safe to use bevacizumab for women who are having surgery and chemotherapy
Who can enter
You may be able to join this trial if all of the following apply. You:
- have been diagnosed with ovarian cancer, fallopian tube cancer or primary peritoneal cancer
- have cancer that is stage 3 or 4
- are well enough to be up and about for at least half the day (performance status 0, 1 or 2)
- have satisfactory blood test results
- are willing to use reliable contraception during the trial and for 6 months afterwards if there is any chance you could become pregnant
- are female and at least 18 years old
You cannot join this trial if any of these apply. You:
- have cancer that has spread to your brain or spinal cord (central nervous system)
- have ovarian cancer that is not epithelial, certain rare types of peritoneal cancer (the trial doctor can advise you about this) or a borderline tumour
- have already had chemotherapy, biological therapy or hormone therapy for ovarian cancer
- have had any other cancer in the last 5 years apart from DCIS, carcinoma in situ of the cervix or non melanoma skin cancer that have been successfully treated, or cancer of the womb lining that was stage 1A and low grade
- have ever had radiotherapy to your tummy (abdomen) or the area between your hip bones (pelvis)
- are going to have chemotherapy directly into your abdomen (intraperitoneal chemotherapy)
- are having any other experimental drug
- are due to have surgery within 4 weeks of starting bevacizumab in this trial
- have damage to your nerves (peripheral neuropathy)
- have protein in your urine (proteinuria)
- have had a mini stroke (TIA), stroke or have had bleeding in your brain in the last 6 months
- have high blood pressure not controlled by medication
- have had a heart attack or angina not controlled by medication in the last 6 months
- have heart failure or problems with the rhythm of your heart that is not well controlled
- have any medical condition that causes abnormal bleeding or blood clotting
- have chronic inflammatory bowel disease, a stomach ulcer or a hole (perforation) in the lining of your stomach or bowel
- have widespread cancer in your lower bowel and rectum or your bowel is blocked (obstructed)
- have an abdominal (tummy)
fistula  which is causing problems
which is causing problems - have a broken bone or a wound that isn’t healing
- have unexplained bloating in your abdomen
- are taking 325mg or more of aspirin a day
- need to have a lot of dental work during the time you would be on treatment
- are known to be sensitive to carboplatin, paclitaxel, bevacizumab or their ingredients
- have any other medical condition that the trial doctors think could affect your taking part
- are pregnant or breastfeeding
Trial design
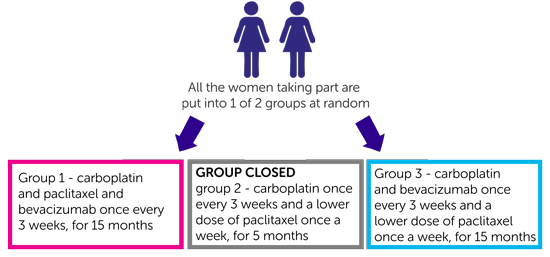
This is a phase 3 trial. It will recruit about 660 women. It is a randomised trial. The people taking part are put into treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in:
- women in group 1 have carboplatin and paclitaxel and bevacizumab once every 3 weeks, treatment lasts a total of 15 months
- women in group 2 have carboplatin once every 3 weeks and a lower dose of paclitaxel once a week, treatment lasts a total of 5 months (Please note: The trial team are no longer putting people into this group.)
- women in group 3 have carboplatin and bevacizumab once every 3 weeks and a lower dose of paclitaxel once a week, treatment lasts a total of 15 months
You have carboplatin, paclitaxel and bevacizumab through a drip into a vein.
Women in groups 1 and 3 then go on to have bevacizumab once every 3 weeks over 39 weeks (13 cycles of treatment).
You may have surgery followed by 6 cycles of chemotherapy. Or you may have 3 cycles of chemotherapy followed by surgery, then 3 more cycles of chemotherapy. But everybody will have chemotherapy for a total of 18 weeks.
If you have surgery during treatment
Women in group 3 will not have any chemotherapy in the third week of the third treatment cycle (the one before surgery). This gives a bit longer to recover before surgery which is a couple of weeks later.
Women in groups 1 or 3 will not have bevacizumab as part of their third treatment cycle. This is because bevacizumab can cause problems with bleeding and healing. You will not have bevacizumab within 4 weeks before or after your surgery.
Everybody will be asked to fill out a questionnaire:
- before starting treatment
- before each cycle of treatment starting with your second cycle
- at follow up appointments after you finish treatment for up to 5 years.
You will also fill in a questionnaire if:
- you are having surgery during treatment you fill it out before your surgery
- your cancer comes back
The questionnaire will ask about any side effects you have had and about how you have been feeling. This is called a quality of life study.
The trial team will ask to take extra blood samples and to take a sample of tissue when you have (or had) surgery. They will look for substances called  in these samples. By looking at these they hope to be able to work out who would benefit most from having weekly chemotherapy.
in these samples. By looking at these they hope to be able to work out who would benefit most from having weekly chemotherapy.
They also want to look for biomarkers that show that a cancer is starting to grow again before it shows on scans. And they want to look at how genes affect ovarian cancer and how people respond to treatment.
You don’t have to give these extra samples for research if you don’t want to. You can still take part in the trial.
Hospital visits
You will see the trial doctors and have some tests before you start treatment. The tests include:
- physical examination
- chest X-ray
- blood tests
- heart trace (
ECG  (electrocardiogram, heart monitor))
(electrocardiogram, heart monitor)) - CT scan or MRI scan
Women in group 1 go to hospital once every 3 weeks to have their chemotherapy treatment.
After the chemotherapy has finished women in the groups having bevacizumab go to hospital once every 3 weeks for treatment.
You have blood tests before every treatment and you see the doctors regularly.
If you are due to have surgery during your treatment you will have a scan before surgery and 4 weeks after surgery. Everybody will have a scan after 6 cycles of treatment and then 16 months after you joined the trial.
After you finish your treatment you see the doctors:
- every 6 weeks for up to 16 months after you joined the trial
- then every 3 months until you have been in the trial for 2 years
- and every 6 months for 6 years after that
Side effects
The possible side effects of carboplatin and paclitaxel include:
- feeling or being sick
- tiredness (fatigue)
- a drop in blood cells causing an increased risk of infection, bleeding problems, tiredness and breathlessness
- hair loss
- aches and pains
- pins and needles or tingling in your hands and feet (peripheral neuropathy)
- ringing in the ears
- mood swings
Some women can have allergic reactions to paclitaxel. To reduce the risk of this you will have steroids and anti-allergy medication before each chemotherapy treatment.
The most common side effects of bevacizumab include:
- raised blood pressure
- slow wound healing
We have more information on
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Andrew Clamp
Supported by
Cancer Research UK
Medical Research Council (MRC)
NIHR Clinical Research Network: Cancer
Other information
This is Cancer Research UK trial number CRUK/13/023.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040



