A trial of a new way to see how well radium 223 is working (REASURE)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at a new way to find out how well radium 223 is working for men with prostate cancer that has spread to the bones. The trial is for men whose prostate cancer is no longer responding to hormone treatment.
More about this trial
Doctors usually treat prostate cancer with hormone therapy. You may also have an operation to remove your testicles. This is called an orchidectomy. These treatments can control your cancer but unfortunately it can start to get worse again and spread. One place in the body prostate cancer can spread to is the bones. If the cancer has spread to more than one place in your bones doctors may use a type of  called radium 223.
called radium 223.
Radium 223 is very similar to  . Like calcium it is taken up by active bone cells. This makes it a good way of targeting cancer cells in the bone. Cancer cells are more active than normal bone cells and so are more likely to pick up the radium 223.
. Like calcium it is taken up by active bone cells. This makes it a good way of targeting cancer cells in the bone. Cancer cells are more active than normal bone cells and so are more likely to pick up the radium 223.
The type of radiation radium 223 uses is called alpha particles. This radiation only travels a short distance, less than a millimetre. This means the cancer cells receive a high dose of radiation and the healthy cells a very small amount or no radiation. So the treatment causes fewer side effects than other types of radiotherapy.
In this trial the researchers want to look at new ways to assess how well the radium 223 is working. This is so doctors can know early on if the treatment is working. They also want to find out if the standard dose is the best dose of radium 223 to use.
The aims of this trial are to
- Find the best way to see how well radium 223 is working for each individual man
- Look at scans and for substances in the blood (
biomarkers  ) that may show how well radium 223 is working
) that may show how well radium 223 is working - Find the best dose to give
Who can enter
You may be able to join this trial if all of the following apply
- Your type of prostate cancer is an adenocarcinoma
- You have had an operation to remove both your testicles (bilateral orchidectomy) or are having long term hormone therapy
- Your PSA blood test level has increased twice in a row and is at least 2 ng/ml (your doctor can tell you this)
- You have a blood test result called ALP that was taken within the last 8 weeks (your doctor can confirm this)
- Your other blood test results are satisfactory
- You have had a
bone scan  in the past 12 weeks that shows at least 2 areas of cancer in your bones
in the past 12 weeks that shows at least 2 areas of cancer in your bones - You are well enough to be up and about for at least half the day (performance status 0, 1 or 2)
- You are at least 18 years old
You cannot join this trial if any of these apply. You
- Have had a type of radiotherapy called radioisotope therapy, for example
strontium 89  (your doctor can tell you about this)
(your doctor can tell you about this) - Have had
chemotherapy  to treat your prostate cancer unless you had it before starting hormone therapy
to treat your prostate cancer unless you had it before starting hormone therapy - Are to start chemotherapy in the next 6 months
- Have had any other anti cancer treatment in the past 4 weeks apart from hormone therapy
- Have cancer that has spread to another part of your body apart from your bones
- Have cancer in your
lymph nodes  and the size of the lymph node is greater than 1½ cm across (your doctor can confirm this)
and the size of the lymph node is greater than 1½ cm across (your doctor can confirm this) - Have had a
scan  that shows you may have, or do have,
that shows you may have, or do have, spinal cord compression  (your doctor can tell this)
(your doctor can tell this) - Have had another cancer in the past 3 years apart from
non melanoma skin cancer  and
and early stage cancer  of the bladder
of the bladder - Have had any experimental drug within 30 days of starting in this trial
- Have had a
blood transfusion  , or a medication called
, or a medication called erythropoietin  , within 4 weeks of starting in this trial
, within 4 weeks of starting in this trial - Are unable to have an MRI scan because you are uncomfortable in small spaces or have certain types of metal surgical clips, metal pins or plates or a pacemaker
- Are not able to control passing a bowel motion (faecal incontinence)
- Have any other medical or mental health condition that the trial team thinks could affect you taking part in this trial
Trial design
This is a phase 2 trial. The researchers need 38 men to join. All the men taking part will have radium 223.
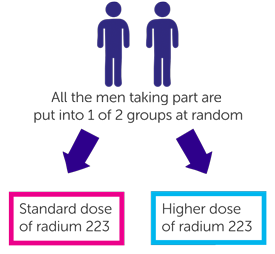
It is a randomised trial. The people taking part are put into treatment groups by a computer. Neither you nor your doctor will be able to decide which group you are in.
- Men in one group will have the standard dose of radium 223
- Men in the other group will have a higher dose of radium 223
You have radium 223 as an injection into a vein through a small plastic tube (cannula) every 4 weeks. Each treatment takes about a minute to give. As long as it is helping and the side effects aren’t too bad you can have up to 6 treatments.
The researchers will ask for a sample of your cancer that was removed when you had surgery or a  , some extra blood samples and urine samples.
, some extra blood samples and urine samples.
They will also ask you to have 3 bone marrow tests. These bone marrow tests are optional. You can agree to have none, 1, 2 or all 3.
The researchers will use all these samples to look for substances ( ) that may show which men may benefit most from having radium 223 in the future.
) that may show which men may benefit most from having radium 223 in the future.
The trial team will use these samples and scans, such as an MRI scan and PET-CT scans, to look for new ways to assess how well the radium 223 is working.
The trial team may ask you to join a sub study. This is to look at 2 different types of  used for a PET-CT scan. They want to find out which one is best to use. You don’t have to agree to take part in this sub study. You can still take part in the main trial.
used for a PET-CT scan. They want to find out which one is best to use. You don’t have to agree to take part in this sub study. You can still take part in the main trial.
Hospital visits
You see the doctor to have some tests before taking part in the trial. These tests include:
During treatment you see the doctor every 4 weeks for the same tests. You have an MRI scan and 2 PET-CT scans before your 1st, 2nd and 4th treatments and then 4 weeks after your last treatment.
The PET-CT scans cannot be done on the same day so you will need to make 2 or more separate visits to the hospital. Your Doctor or Nurse will arrange for this in advance.
If you agreed to have the bone marrow tests, you have the tests done before your 1st and 2nd treatments and then 4 weeks after your last treatment.
You see the doctor 4 months after finishing treatment and then every 4 months for a year for a physical examination and blood tests.
Side effects
The most common side effects of radium 223 are
- Diarrhoea
- A drop in blood cells causing an increased risk of infection, bruising and bleeding
After treatment some radiation may be in your urine for a few hours or your poo (faeces) for up to a week. The total amount is extremely small and gets smaller each day. Your nurse or doctor will tell you about some special precautions you need to take.
The trial doctor will also talk to you about the possible side effects of radium 223 before you agree to take part in the trial.
We have information about radium 223.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Christopher Parker
Supported by
Bayer
Institute of Cancer Research (ICR)
NIHR Clinical Research Network: Cancer
The Royal Marsden NHS Foundation Trust
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040