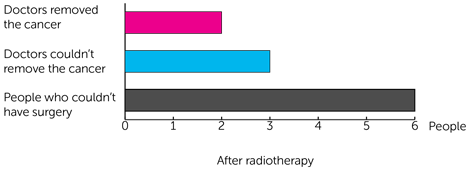A trial looking at stereotactic body radiotherapy before surgery for pancreatic cancer (SPARC)
Cancer type:
Status:
Phase:
This trial looked at whether stereotactic body radiotherapy (SBRT) could help shrink pancreatic cancer before surgery to remove it. It was for people with cancer that doctors hoped they could remove with surgery, but the operation was likely to be difficult.
The trial was supported by Cancer Research UK. It was open for people to join between 2015 and 2018. The team published the results in 2021.
More about this trial
Doctors treat pancreatic cancer with surgery if they can. But sometimes this is difficult because of the size or position of the cancer. It may be close to a large blood vessel, for example.
Researchers wanted to find out if stereotactic body radiotherapy (SBRT) could help shrink the cancer. This would help make the operation easier and more successful.
With SBRT, radiotherapy beams are directed at the cancer from different positions around the body. So it accurately delivers a high dose of treatment to the area of cancer. And a very low dose to surrounding healthy tissue. This helps prevent side effects.
The main aims of this trial were to find out:
- the best dose of SBRT to use
- whether having SBRT before surgery can improve the chances of removing all the cancer
- more about the side effects
Summary of results
The research team found that stereotactic body radiotherapy (SBRT) didn’t cause too many side effects. But it was hard to draw any firm conclusions because only a small number of people joined the trial.
Trial design
This trial was for people with pancreatic cancer that the doctors thought they may be able to remove with surgery. But the operation would be difficult.
Everyone taking part was due to have SBRT. The team planned to look at 4 different doses of radiotherapy. This was so the they could work out the best dose to give.
But they found it harder than they expected to find people to join the trial. So they looked at 2 different doses. The trial closed earlier than planned because fewer people joined the trial than they’d hoped.
Results
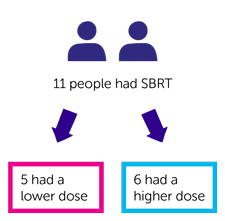
A total of 12 people joined the study, and 11 had SBRT as planned.
The first 5 people who had treatment had a lower dose of radiotherapy. The next 6 people who had treatment had a higher dose of radiotherapy.
After radiotherapy:
- 2 people had surgery and doctors were able to remove the cancer
- 3 people had surgery but the doctors weren’t able to remove all of the cancer
- 1 person wasn’t able to have surgery because of the concerns about them having an anaesthetic
- 5 people weren’t able to have surgery because their cancer had grown
Side effects
Most of the people who took part had at least 1 side effect. But many were mild or didn’t last long.
Nearly half the people taking part reported having at least 1 side effect that affected their digestive system. This included feeling or being sick, pain or heartburn. Most of these were mild or didn’t last long. And it’s difficult for the team to know for sure whether they were side effects caused by treatment, or symptoms of the cancer itself.
No one had side effects serious enough to suggest that the dose of radiotherapy was too high.
Conclusion
The trial team found it hard to draw any firm conclusions because of the small number of people who took part. But they found that the doses of SBRT used in this trial didn’t cause too many side effects.
Following this and other trials, in 2021 NHS England recommended SBRT for people with pancreatic cancer that cannot be removed with surgery but has not spread.
Where this information comes from
We have based this summary on information from the research team. The information they sent us has been reviewed by independent specialists ( ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Maria A. Hawkins
Supported by
Cancer Research UK
Experimental Cancer Medicine Centre (ECMC)
Cancer Research UK Oxford Centre
CRUK/MRC Oxford Institute for Radiation Oncology
Oncology Clinical Trials Office (OCTO)
Oxford Clinical Trials Research Unit (OCTRU)
University of Oxford
National Institute for Health Research (NIHR)
Other information
This is Cancer Research UK trial number CRUK/14/028.
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040