A trial of CAR T-cell treatment and pembrolizumab for diffuse B cell lymphoma (ALEXANDER)
Cancer type:
Status:
Phase:
This trial looked at changing immune cells to recognise and attack diffuse B cell lymphoma. The people taking part had their own immune cells called  changed. This treatment is called CAR T-cell treatment.
changed. This treatment is called CAR T-cell treatment.
The trial was for people with diffuse B cell lymphoma (DBCL) who had not had CAR T-cell treatment before. And their lymphoma had come back after treatment, or treatment had stopped working.
The trial was open for people to join between 2017 and 2021. The team published the results in 2023.
More about this trial
There are several treatments for DBCL, including  . But in some people, the lymphoma comes back, or treatment stops working. So, doctors are looking for new treatments to help people in this situation. In this trial they were looking at:
. But in some people, the lymphoma comes back, or treatment stops working. So, doctors are looking for new treatments to help people in this situation. In this trial they were looking at:
- CAR T-cell treatment
- pembrolizumab
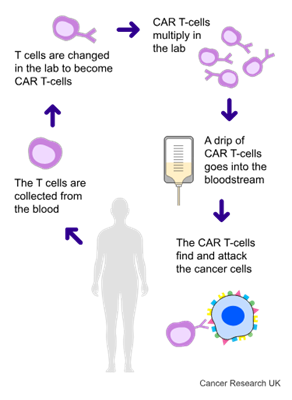
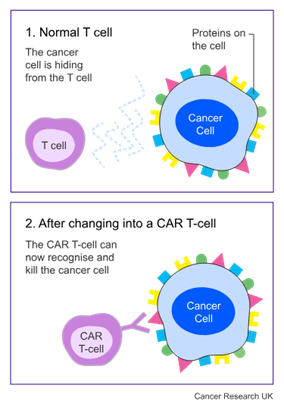
CAR T-cell treatment uses your own T cells. They are part of your  . They are good at fighting infection, but they aren’t good at recognising lymphoma cells. With this treatment, the doctor takes some T cells from your blood and in a laboratory changes them using a harmless virus. This is when they become CAR T-cells.
. They are good at fighting infection, but they aren’t good at recognising lymphoma cells. With this treatment, the doctor takes some T cells from your blood and in a laboratory changes them using a harmless virus. This is when they become CAR T-cells.
Once they have done this, they give the changed T cells back to you. This helps the T cells to recognise and find lymphoma cells.
At the time of this trial, CAR T-cell treatment targeted 1 protein on the surface of the cell. But sometimes this treatment doesn’t work for very long. Researchers think this might be because the lymphoma cells can hide away and not be recognised.
So, in this trial, they looked at CAR T-cell treatment that targets 2 proteins. These proteins are CD19 and CD22. It’s also called AUTO3 treatment.
Pembrolizumab is a type of  . It blocks a protein called PD-1. This triggers the immune system to attack and kill lymphoma cells. Doctors wanted to see if it helped the CAR T-cells to work better.
. It blocks a protein called PD-1. This triggers the immune system to attack and kill lymphoma cells. Doctors wanted to see if it helped the CAR T-cells to work better.
This was a phase 1/2 trial in two parts.
Part 1
In part 1 researchers were looking at the best dose of CAR T-cells for people to have.
Part 2
In part 2 the researchers tested this dose in more people. Part 2 started when they found the best dose in part 1.
The main aims of the trial were to find out:
- how safe treatment is
- how well treatment works
- if it is possible for some people to have treatment as an outpatient
- more about the side effects
Summary of results
The trial team found that they could give the treatment safely, and some people were able to have it as an outpatient. This meant that, on average, they spent 2 weeks less in hospital.
The trial team found that in around 2 out of 3 people taking part the lymphoma had either:
- shrunk by at least one third (30%), this is called a
partial response  or
or - gone away this is called a
complete response 
This was for at least some time.
In some cases, the lymphoma got worse or came back again.
The team are not sure if the pembrolizumab helped the CAR T-cells work better. They would need to do more research to find out for sure.
Results
62 people had their AUTO3 CAR T-cells made for them. And 52 people had treatment. 10 people didn’t have their treatment, either because their lymphoma got worse, or they died, before it was available.
48 people also had pembrolizumab. Some people had pembrolizumab when they were having chemotherapy to prepare for the CAR T-cells. Other people had pembrolizumab after their CAR T-cell infusion.
When the team published these results they had monitored people after treatment, on average, for nearly 22 months. They were able to look at how well the treatment worked in 47 people. They found that:
- the lymphoma went away completely in 23 people, this is a complete response
- the lymphoma shrunk, and there were no new areas of lymphoma, in 8 people, this is a partial response
The trial team looked at the time from when the treatment started working to when the lymphoma came back. This is the duration of response. They found this was 8.3 months on average for everyone who had treatment. But in the people whose lymphoma went away completely more than half showed no signs of lymphoma after 12 months.
Although the treatment worked well at controlling the cancer at first, this did not always last. Out of the 23 people whose lymphoma went away completely, 13 people’s lymphoma came back (relapsed).
The researchers looked at how long, on average, it was until half the people taking part were still alive. This is called median overall survival. They found this was 13.8 months after treatment. The researchers say this compares to about 4.4 months in people in a similar situation who have  .
.
Side effects
The treatment did cause some side effects, but most of these were mild and did not cause too many problems.
The main side effects were:
cytokine  release syndrome (CRS). Symptoms include fever (high temperature), dizziness due to low blood pressure or difficulty breathing.
release syndrome (CRS). Symptoms include fever (high temperature), dizziness due to low blood pressure or difficulty breathing.- infection
- moderately low
neutrophil  level
level - moderately low
platelet  level
level
Some people had more severe side effects. These included:
- one person had severe cytokine release syndrome
- two people had severe side effects affecting the brain (neurotoxicity)
- two people developed haemophagocytic lymphohistiocytosis (HLH). This is a rare condition where the body produces too many white blood cells called lymphocytes and histiocytes. If it is not treated, these extra cells collect in organs in the body and damage them.
Conclusion
The researchers concluded that the side effects of AUTO3 and pembrolizumab were tolerated well. And people were able to have treatment as an outpatient.
The trial team say that AUTO3 does not prevent the lymphoma coming back in enough people. They think more research is needed to look at improving AUTO3 in the future.
More detailed information
There is more information about this research in the references below.
Please note, the information we link to here is not in plain English. It has been written for healthcare professionals and researchers.
Dual targeting of CD19 and CD22 with bicistronic CAR-T cells in patients with relapsed/refractory large B-cell lymphoma
C Roddie and others
Blood, 2023. Volume 141, issue 20, pages 2470–2482
890MO Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR.T cell, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL (Abstract)
E Thoulani and others
Annals of Oncology, 2020. Volume 31, supplement 4
Where this information comes from
We have based this summary on the information in the articles above. These have been reviewed by independent specialists ( ) and published in medical journals. We have not analysed the data ourselves. As far as we are aware, the links we list above are active and the articles are free and available to view.
) and published in medical journals. We have not analysed the data ourselves. As far as we are aware, the links we list above are active and the articles are free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Kirit Ardeshna
Supported by
Autolus Ltd
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040