A trial looking at RSO-021 for fluid around the lung caused by cancer (MITOPE)
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
Cancer type:
Status:
Phase:
This trial is looking at a new drug called RSO-021 for fluid around the lung caused by cancer.
It is for people who have any type of solid cancer including mesothelioma of the lung.
Solid tumours are any type of cancer apart from blood cancers such as leukaemia.
More about this trial
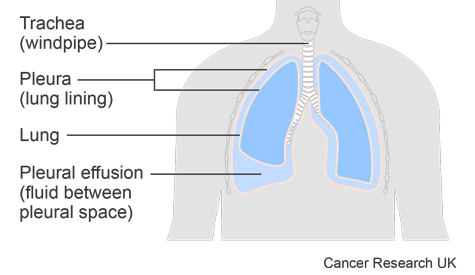
Cancer can cause fluid to collect around the lungs. This is called a pleural effusion or a malignant pleural effusion. There are two sheets of tissue that protect the lungs. These sheets are called pleura and there is a space between the pleura called the pleural space. The build up of fluid in this space stops your lungs from expanding fully. So you have to take shallower breaths and make more effort to breathe. Doctors are looking at ways to improve treatment for this condition.
In this trial they are looking at a drug called RSO-021. It targets an enzyme called PRX3 in cancer cells. Targeting this enzyme aims to kill the cancer cells without affecting normal cells. This is the first time people are having thiostrepton. You have thiostrepton through a tube (catheter) into the pleural space.There are 2 parts to this trial. Part 1 looked at the best dose of RSO-021 to give. This part is now closed to recruitment. Part 2 is testing this dose in more people. Part 2 is open.
Everyone in the trial has RSO-021. A few people also have a chemotherapy drug called paclitaxel. This is a  for some cancer types. Your doctor will let you know if you have this.
for some cancer types. Your doctor will let you know if you have this.
The aim of this trial is to find out:
- the best dose of RSO-021 to give
- how safe it is
- how well it works
- more about the side effects
Who can enter
The following bullet points are a summary of the main entry conditions for this trial. Talk to your doctor or the trial team if you are unsure about any of these. They will be able to advise you.
Who can take part
You may be able to join this trial if all of the following apply:
- You have a solid tumour including
mesothelioma  of the lung.
of the lung. - You have a malignant pleural effusion that is caused by your cancer.
- You have had at least one treatment for solid cancer or mesothelioma that has spread elsewhere in the body but your cancer is getting worse. Or you have mesothelioma and you haven’t had treatment yet.
- There is a sample of tissue (
biopsy  ) the team can access or you are willing to give a new sample. And you are willing to give extra samples of tissue during the trial. This is only for certain groups. Your doctor will talk to you more about this.
) the team can access or you are willing to give a new sample. And you are willing to give extra samples of tissue during the trial. This is only for certain groups. Your doctor will talk to you more about this. - The build up of fluid is mainly in the pleural space and your doctor thinks they can put a tube through your skin into this space to give you treatment with RSO-021.
- You are fit and active but might not be able to do heavy physical work (performance status 0 to 1).
- You have satisfactory blood test results.
- You are willing to use reliable contraception during the trial and for a period after if there is any chance you or your partner could become pregnant.
- You are at least 18 years old.
Who can’t take part
Cancer related
You cannot join this trial if any of these apply. You:
- have cancer that has spread to the brain or spinal cord or the tissues around the brain. You may be able to take part if the cancer is stable and you aren’t taking steroids.
- have had cancer treatment to the whole body (
systemic treatment  ) within 3 weeks of joining the trial or the treatment hasn’t cleared your body yet
) within 3 weeks of joining the trial or the treatment hasn’t cleared your body yet - have had radiotherapy to the chest or major surgery within 3 weeks of joining the trial. You can take part if you had radiotherapy to treat symptoms (palliative radiotherapy) that cancer can cause.
- have a large area of cancer near the lungs or areas of fluid around the lung which means it isn’t possible to give RSO-021. Your doctor will know this.
- have had an experimental treatment or used an experimental device within 30 days of starting trial treatment
- have another cancer or had another cancer in the past that could affect you taking part in the trial
Medical conditions
You cannot join this trial if any of these apply. You:
- have HIV, an active hepatitis B or hepatitis C infection or any other infection that isn’t controlled with medication
- are taking medication to thin the blood because you had a blood clot. You can join if you are having this type of medication to prevent a clot from forming. This is only if it won’t interfere with having the tube put into your chest or giving tissue samples. Your doctor can tell you more about this.
- have had
steroid treatment  for an inflammatory condition or
for an inflammatory condition or autoimmune disease  within 15 days of starting trial treatment. Or you have had another treatment that damps down the immune system within 3 weeks of starting trial treatment. You can take part if you are having low doses of steroids. Your doctor will know this.
within 15 days of starting trial treatment. Or you have had another treatment that damps down the immune system within 3 weeks of starting trial treatment. You can take part if you are having low doses of steroids. Your doctor will know this. - have side effects due to previous treatments that aren’t getting better. You can take part if you have hair loss.
- have any other medical condition or mental health problem that could affect you taking part
Other
You cannot join this trial if any of these apply. You:
- are allergic to RSO-021 or anything that it contains
- have a problem with drugs and alcohol
- are pregnant or breastfeeding
- have had a live
vaccine  within 4 weeks of joining the trial. Please note that the approved COVID-19 vaccines are allowed as they aren’t live.
within 4 weeks of joining the trial. Please note that the approved COVID-19 vaccines are allowed as they aren’t live.
| As well as the above there are specific entry conditions and exclusion criteria for each dose expansion group. Speak to your doctor or research nurse if you want to find out more about the entry conditions for this trial. |
Trial design
This phase 1/2 trial is taking place in the UK. The team need 30 people to join part 1 and 84 people to join part 2.
This trial is in 2 parts:
- dose escalation (part 1) – this part is now closed to recruitment
- dose expansion (part 2) - this part is open for people to join
Dose escalation (part 1)
This part is now closed. The team found the best dose of RSO-021 to give.
Dose escalation (part 2)
Part 2 is open. In this part the researchers test the best dose of RSO-021 found in part 1.
The group you join depends on your cancer type and if you have already had treatment. Your doctor can tell you more about this.
There are 4 groups in the dose escalation part:
- group 1 is only for people who have a solid tumour but you can’t join if you have
mesothelioma  . You have RSO-021.
. You have RSO-021. - group 2 is only for people who have breast cancer,
ovarian cancer  or
or non small cell lung cancer  . You have RSO-021 and a chemotherapy drug called paclitaxel.
. You have RSO-021 and a chemotherapy drug called paclitaxel. - group 3 is only for people with mesothelioma. You have RSO-021.
- group 4 is for people with mesothelioma who haven’t yet had treatment or don’t want to have it. And who have a recent diagnosis of a pleural effusion. You have RSO-021.
Trial treatment and how you have it
The trial team arrange for you to have a small plastic tube put under the skin into the pleural space in your chest. This is called an indwelling pleural catheter (IPC).
You have a  to numb this area before they put the tube in. Your doctor will explain in more detail what happens during the procedure. The tube stays in place during the trial. You have RSO-021 through this tube. It takes about 15 to 30 minutes each time. You might be at the hospital for longer.
to numb this area before they put the tube in. Your doctor will explain in more detail what happens during the procedure. The tube stays in place during the trial. You have RSO-021 through this tube. It takes about 15 to 30 minutes each time. You might be at the hospital for longer.
You have RSO-021 as long as it is working and the side effects aren’t too bad.
People in group 2 of the dose expansion part have RSO-021 and paclitaxel chemotherapy. You have paclitaxel as a drip into a vein. You have it once every 3 weeks.
Samples for research
The team ask to take some extra blood samples. Where possible, you have these at the same time as your routine blood tests. They would also like to take samples of fluid from your lung. They take these using the tube in your chest.
They might also ask for an extra tissue sample.
The team plan to use the samples to:
- find out what happens to RSO-021 in the body
- look at
genes  to understand more about cancer and better understand how RSO-021 works
to understand more about cancer and better understand how RSO-021 works - look for substances called
biomarkers 
If you prefer, you can say no to the team using your samples to look for genes and biomarkers. You can still join the main part of the trial.
Hospital visits
You see the doctor and have tests before you can take part. These include:
- blood tests
- a
physical examination 
- heart trace (
ECG  )
) - chest x-ray
- urine samples
- CT scan or MRI scan
- PET-CT scan
You have treatment at the hospital in the outpatient department. Some of the hospital visits may be longer if the team are taking extra blood samples or fluid samples from the lung. The team can tell you more about this.
During treatment you see the doctor regularly. This is for blood tests and to see how you are.
Trial scans
You have an MRI scan, CT scan or PET-CT scan every 6 weeks.
Follow up visits
You see the trial team every 3 months when you stop treatment. At each visit you have a check up and blood tests. You also have a scan as part of this trial if you stopped treatment but your cancer hasn’t got worse.
In some cases, the team might call you to see how you are getting on.
Side effects
The trial team monitor you during treatment and afterwards. Contact your advice line or tell your doctor or nurse if any side effects are bad or not getting better.
This is the first time people are having RSO-021. So we don’t know what all the side effects are. The possible side effects we know about so far include:
- areas of inflammation in the sheets of tissue that cover the outside of the lung and the lining of the chest cavity (pleura)
- a build up of
white blood cells  in the 2 layers of pleura (pleural space)
in the 2 layers of pleura (pleural space) - low blood pressure
- shortness of breath
- pain in the chest (pleural pain)
- constipation
We have more information about paclitaxel and its side effects.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor James Spicer
Supported by
RS Oncology LLC
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040