A study of active surveillance, aspirin and vitamin D in men with prostate cancer (PROVENT)
Cancer type:
Status:
Phase:
This study was for men who were having regular monitoring for prostate cancer. It was open to men who had  .
.
This trial was open for people to join between 2016 and 2017. The team published the results in 2022.
More about this trial
Prostate cancer can grow slowly and may never cause symptoms. So some men with localised prostate cancer have regular monitoring. This is active surveillance.
This means you don’t have treatment straight away. Your doctor keeps a close eye on you to check for any signs that the cancer is growing. If it does you and your doctor can decide which treatment is best for you.
Research suggests that having aspirin and vitamin D can stop cancer from growing and changing.
This was a  . This type of study helps the team check the design before running a larger trial. The aims of this study were to find out:
. This type of study helps the team check the design before running a larger trial. The aims of this study were to find out:
- how willing men are to take part
- how well this treatment works for prostate cancer that has a low risk of growing or spreading
Summary of results
The team found that this type of trial was acceptable and safe to do.
Results
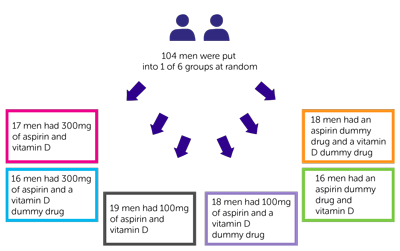
This was a feasibility study. 130 men met the conditions to take part in the trial. Of these 104 men decided to take part.
It was a  study. A computer put the 104 men into 1 of 6 groups. Neither they nor their doctor chose which group they went into.
study. A computer put the 104 men into 1 of 6 groups. Neither they nor their doctor chose which group they went into.
Of the 104 men:
- 17 men had 300mg of aspirin and vitamin D
- 16 men had 300mg of aspirin and a vitamin D dummy drug
- 19 men had 100mg of aspirin and vitamin D
- 18 men had 100mg of aspirin and a vitamin D dummy drug
- 16 men had an aspirin dummy drug and vitamin D
- 18 men had an aspirin dummy drug and a vitamin D dummy drug
The study was set up to find out if it was possible to run a larger trial. This suggests that running a larger trial would be possible.
The team looked at the percentage of men who took their medication as directed. They found that 89 out of every 100 men (89%) did so during the study.
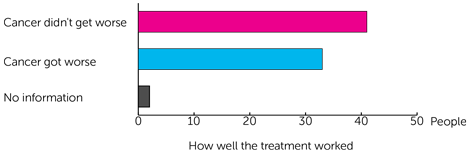
They were able to look at how well the treatment had worked in 76 of the men who took part. After a year they found that the:
- cancer didn’t get worse in 41 men
- cancer did get worse in 33 men
For 2 of the men the information wasn't available.
During the study 18 men decided to stop taking part in the study:
- 9 decided to have radiotherapy or surgery to remove their tumour
- 7 stopped due to reasons not related to their prostate cancer or treatment
- 2 men stopped due to side effects. For 1 man it was a very serious side effect.
In total there were 11 side effects. Of these 6 were not serious and 5 were.
Bleeding from the back passage was one of the serious side effects. This occurred twice in 1 man. His doctor thought the treatment caused it. He was taking 100mg aspirin and a vitamin D tablet. He stopped taking the treatment and no longer took part in the study.
Conclusion
The team concluded that their feasibility study showed that it was acceptable and safe to do a trial like this. They noted that another similar trial was to open using a different type of drug.
They said that researchers need to do a larger  to work out what are the best drugs to use.
to work out what are the best drugs to use.
More detailed information
There is more information about this research in the reference below.
Please note, this article is not in plain English. It has been written for healthcare professionals and researchers.
Journal articles
Feasibility of aspirin and/or vitamin D3 for men with prostate cancer on active surveillance with Prolaris® testing
Eoin Dinneen, Gregory L. Shaw and others
BJUI Compass. 2022. Volume 3. Pages 458–465.
Where this information comes from
We have based this summary on the information in the article above. This has been reviewed by independent specialists ( ) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
) and published in a medical journal. We have not analysed the data ourselves. As far as we are aware, the link we list above is active and the article is free and available to view.
Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Greg Shaw
Supported by
Barts and the London Charity
Experimental Cancer Medicine Centre (ECMC)
Queen Mary University of London
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040