A study looking at using a detailed type of MRI scan to help diagnose prostate cancer (PROMIS)
Cancer type:
Status:
Phase:
This study was to see how useful a type of MRI scan called multi parametric (MP) MRI was to diagnose prostate cancer.
More about this trial
- see how well MP MRI showed which people didn’t need a biopsy
- see whether MP MRI helped to find cancers that didn’t need treatment
- work out how cost effective MP MRI was as a test for prostate cancer
Summary of results
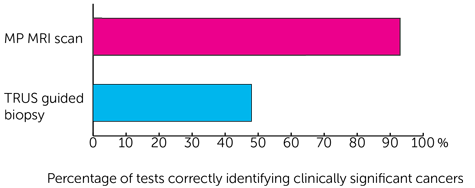
 of greater than or equal to 4+3 or was at least 6mm in length.
of greater than or equal to 4+3 or was at least 6mm in length.- 93 out of every 100 times (93%) for the MP MRI scan
- 48 out of every 100 times (48%) for the TRUS guided biopsy
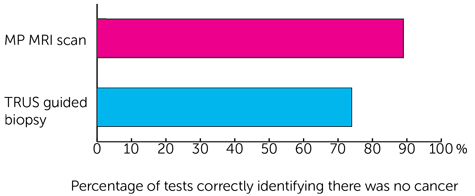
- 89 out of every 100 times (89%) for the MP MRI scan
- 74 out of every 100 times (74%) for the TRUS guided biopsy
 ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Professor Mark Emberton
Supported by
Experimental Cancer Medicine Centre (ECMC)
Medical Research Council (MRC)
NIHR Clinical Research Network: Cancer
NIHR Health Technology Assessment (HTA) programme
University College London (UCL)
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040