Please note
This trial is no longer recruiting patients. We hope to add results when they are available.
Brain (and spinal cord) tumours
Closed
Other
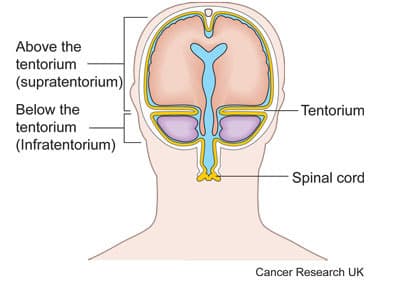
This study is to see if a PET-CT scan and magnetic resonance spectroscopy (MRS) can help doctors make an assessment of brain tumours. The study is open to people with a type of brain tumour called a glioma that is above the hind brain (cerebellum) and brain stem. Doctors call this a supratentorial glioma.
Doctors use magnetic resonance imaging (MRI) scans to diagnose brain tumours. They are best at finding out the size of the tumour and where it is. But they don’t give doctors all the information they would like.
Magnetic resonance spectroscopy (MRS) scans are similar to MRI scans, but can give extra information. MRS scans may be able to give information about how quickly your tumour is growing and if certain treatments will work.
In this study, they are also looking at PET-CT scans. This scan combines a PET scan and CT scan into one scan.
Before having a PET-CT scan, you have an injection of a small amount of radioactive drug called a tracer. In this study the researchers are using a tracer that may give doctors more information about your tumour and how fast it is growing.
The researchers will compare the results of these 2 scans with other tests done on a sample of your tumour tissue. The main aim is to find out how good MRS and PET-CT scans are for assessing gliomas.
Recruitment start: 18 December 2014
Recruitment end: 31 March 2016
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Dr Adam Waldman
Imperial College London
NIHR Biomedical Research Centres (BRCs) Award
NIHR Clinical Research Network: Cancer
Last reviewed: 30 Mar 2016
CRUK internal database number: 12771