Last reviewed: 17 October 2025
Last reviewed: 17 October 2025
The International Cancer Benchmarking Partnership (ICBP) is a unique and innovative collaboration that brings together clinicians, policymakers, researchers and data experts across the world. It aims to measure international variation in cancer survival, incidence and mortality, as well as identify factors that might be driving these differences.
The ICBP produces high quality research to help identify best international practice, and generate insights needed for policy and practice change. This will help to enable optimisation of cancer services and improvement of outcomes for cancer patients.
The ICBP was set up in 2009 to deliver high quality, comparative research on cancer survival, incidence and mortality trends across high income countries. Our research uses a range of data sources to explore factors that could explain international variation in survival rates.
The partnership provides the opportunity to compare data and share practices and policies that seem to be contributing to improved cancer outcomes. The ICBP wants to ensure patients across the globe experience the best possible cancer services and aims to drive international improvement in cancer registry practices.
Phase 1 established benchmarks for breast, colorectal, lung, and ovarian cancers, comparing survival, mortality, incidence and stage data from 1995–2007 to quantify international differences. Exploratory modules focused on public awareness and beliefs about cancer, the role of primary care in timely referral, measuring time intervals from symptoms to diagnosis and treatment, and the impact of registry processes and comorbidities on short-term outcomes.
The Phase 2 and Transition phase benchmark included 8 cancer sites: colon, liver, lung, oesophagus, ovarian, pancreas, rectal, and stomach. Phase 2 investigated access to primary care and post diagnostic tests, access to optimal treatment, cancer patient pathways, and the organisation and structure of health systems. In 2021, the partnership entered a 2-year Transition Phase where it commissioned two research projects to assess COVID-19’s impact on cancer incidence and services
Phase 3 will include 10 cancer sites, with an updated benchmark that will include an expanded focus on inequalities in cancer outcomes where possible, alongside a continued emphasis on data quality, completeness, and comparability. Our four exploratory modules aim to deepen insights into treatment, cancer care pathways, cancer workforce, and models of care.
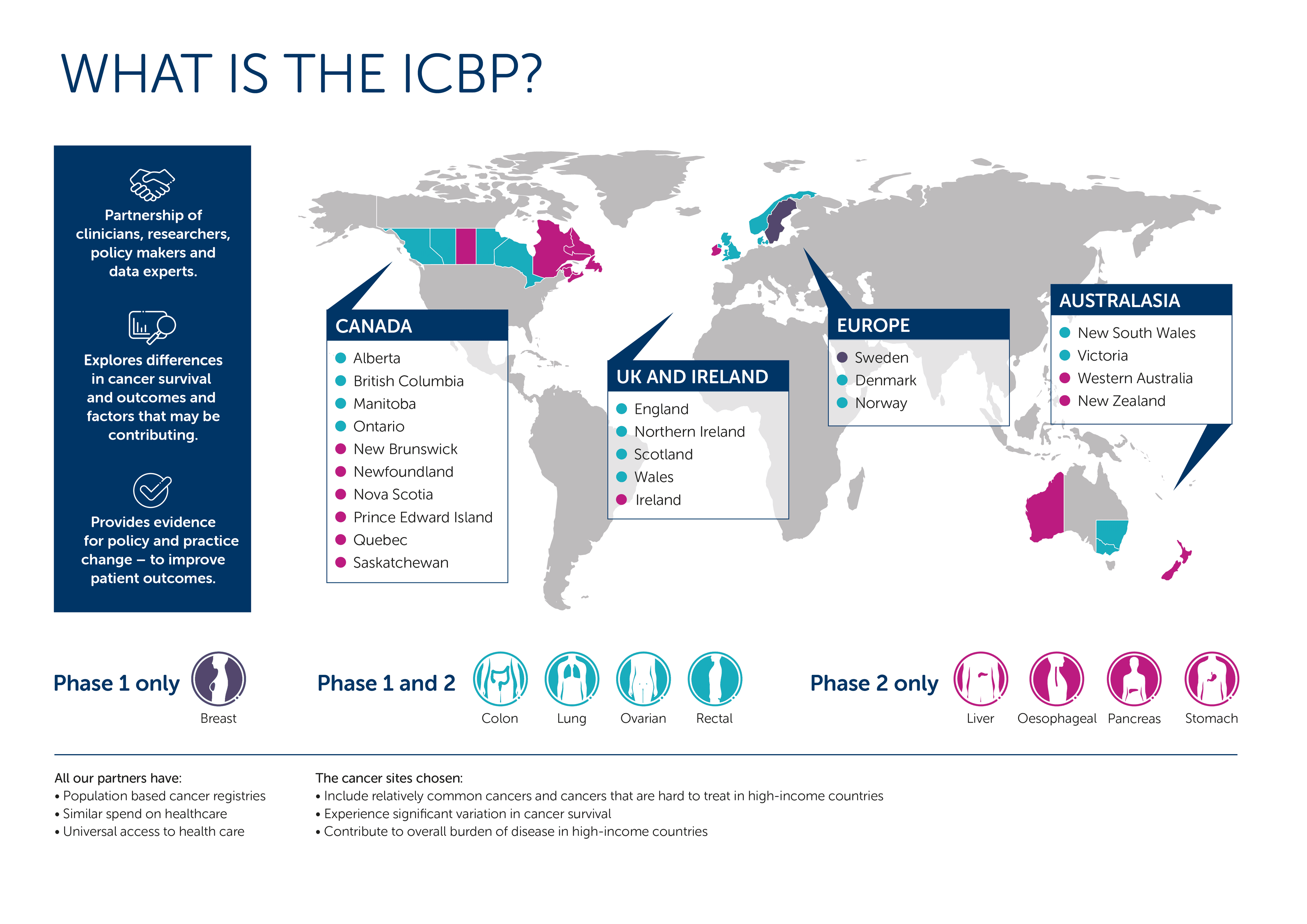
The partnership includes 24 jurisdictions across 9 countries and 3 continents, including:
Australia: New South Wales, Queensland, Victoria and Western Australia.
Canada: Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland, Nova Scotia, Ontario, Prince Edward Island, Quebec and Saskatchewan.
Denmark
Ireland
New Zealand
Norway Sweden (phase 1 only)
The United Kingdom: England, Northern Ireland, Scotland and Wales.
The United States (The US Healthcare Delivery Research Program has joined as an associate member for Phase 3. Associate members participate in some of the ICBPs research modules but not the benchmark. They may also not meet all the criteria of full members).
All full member Jurisdictions of the ICBP have:
High quality and long-standing population-based cancer registration with good coverage, to ensure that cancer survival results represent the entire population.
Primary care led and universal access to health care – for which there will be similar policy considerations.
Broadly comparable wealth and similar expenditure on healthcare provision.
In Phase 3, we are focusing on 10 cancer sites:
Breast, Colon, Lung, Ovarian, Rectal, Liver, Oesophageal, Pancreas, Stomach, Cervical
The cancer sites studied by the ICBP:
Include relatively common cancers and cancers where similar challenges are faced across partner countries.
Observed international variation in cancer survival.
Contribute significantly to overall burden of disease in high-income countries.

The ICBP is led by the Programme Board, currently chaired by Mark Lawler, Associate Pro-Vice-Chancellor, Professor of Digital Health, and Chair in Translational Cancer Genomics at Queen’s University Belfast, with support from Deputy Chair David Cameron, Professor of Medical Oncology at the University of Edinburgh.
The Programme Board serves as the key decision-making body, meeting quarterly and including funder representatives from each participating jurisdiction. Its diverse expertise—clinical, academic, and policy-focused—ensures a comprehensive and effective approach to ICBP activities.

The ICBP has an expert advisory group that reviews outputs, supports translation of the evidence into local contexts and provides advocacy of our research to different audiences. These groups focus on one of three streams: The Advisory Group comprises a ‘core’ group of members who provide Research, Policy, or Clinical advice and is designed to support different aspects of the journey of ICBP research, from scoping and designing research projects, to ensuring clinical expertise are embedded and advocating for policy change. This is complemented by specialist expertise for specific cancer sites, modalities and wider health system understanding for specific pieces of research and application.
John Butler, Consultant Gynaecological Oncology Surgeon at the Royal Marsden, is the Lead Clinical Advisor to the ICBP and Chair of the Advisory Group.
The Programme Management Team (PMT) have two roles, to facilitate the delivery of all programme activities and to conduct the in-house research. The PMT are hosted by Cancer Research UK, a partner organisation of the ICBP.
If you have any views or comments about the partnership, we would like to hear from you.
Join the conversation and follow the ICBP on LinkedIn to stay informed on the latest updates from the partnership.
Join the conversation on LinkedIn