All cancer types
Results
Other
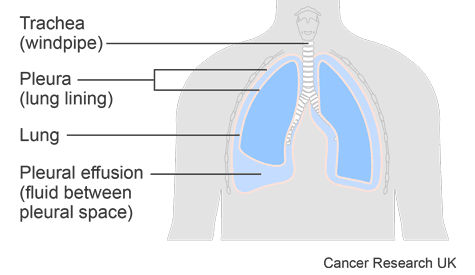
This trial was for people who had a pleural effusion caused by cancer. A pleural effusion is a build up of fluid in the space between the sheets of tissue that cover the outside of the lung and the lining of the chest cavity. These sheets of tissue are called the pleura.
The trial was open for people to join between 2016 and 2019. The team published the results about ultrasound scans in 2022. And the results about in 2018.
Recruitment start: 1 January 2016
Recruitment end: 31 December 2019
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Professor Najib Rahman
European Respiratory Society
Marie Curie Cancer Care
NIHR Clinical Research Network: Cancer
Slater & Gordon Research Fund
University of Oxford
Last reviewed: 02 Sept 2024
CRUK internal database number: 11773