A study looking at AZD5363 for cancer (OAK)
Cancer type:
Status:
Phase:
This study looked at capivasertib (AZD5363) for solid tumours that continued to grow despite all other standard treatments.
More about this trial
- how well the tablet worked
- how safe the tablet is
- if it was better to take capivasertib with food or not
Summary of results
The team found that the tablet worked as well as the capsule and that the side effects were generally the same as the capsule.
About this study
This was a phase 1 study. There were 2 parts to this study.
When part A was completed another group of people were asked to join part B.
In part A the team looked at how well the tablet and the capsule were absorbed by the body.
In part B the team wanted to know if having capivasertib with food affected how well it was absorbed by the body. They did this by measuring the amount of capivasertib in the blood over a certain period of time.
Results of part A
18 people took part. Everyone took the capivasertib tablet for 1 week and then they had the capsule for the following week.
In both weeks, they took capivasertib in the morning and the evening for 4 days. And then they had no treatment for 3 days. They took it on an empty stomach. That is no food from 2 hours before taking the tablet and no food to 1 hour after. This is the standard way to take capivasertib.
On the 4th day of week 1 and week 2 a number of blood samples were taken before and after taking capivasertib.
The researchers compared the results of the blood samples from week 1 and week 2. This was to find out how well the capivasertib tablet and capsule were absorbed. And what happened to capivasertib in the blood.
They found that the tablet was absorbed faster than the capsule. But the total amount of capivasertib absorbed by the body from the tablet and the capsule and detected in the blood over a certain period of time was similar.
Results of part B
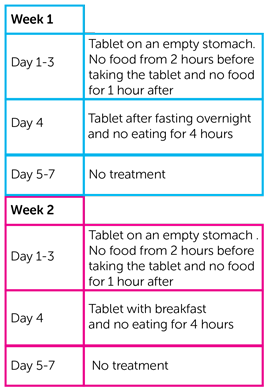
12 people took part. Everyone took capivasertib as a tablet in the morning and the evening for 4 days. And then they had no treatment for 3 days.
In week 1, for 3 days they took the tablet 2 hours before food and no food for 1 hour after taking it. This is the standard way of taking capivasertib.
On the 4th day they took the tablet after fasting overnight (not eating for at least 8 hours). And then they couldn’t eat for 4 hours after taking the tablet.
In week 2, for 3 days they took the tablet according to the standard way.
On the 4th day, they took the morning tablet with their breakfast after fasting overnight. And then they couldn’t have anything to eat for 4 hours after taking the tablet.
Everyone’s breakfast contained the same amount and type of food.
- diarrhoea
- a raised amount of glucose (sugar) in the blood
- feeling sick
- diarrhoea
- a raised amount of glucose in the blood
- a drop in red blood cells
- a high temperature
- diarrhoea
- skin rash
- absorbed by the body in a similar way and time
- the side effects were similar
 ) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.
) and published in a medical journal. The figures we quote above were provided by the trial team who did the research. We have not analysed the data ourselves.Recruitment start:
Recruitment end:
How to join a clinical trial
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Chief Investigator
Dr Udai Banerji
Supported by
AstraZeneca
Experimental Cancer Medicine Centre (ECMC)
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040