
"He went through six operations and was placed on a clinical trial so he could try new treatments.”
Please note - this trial is no longer recruiting patients. We hope to add results when they are available.
This study is looking at using the results of laboratory tests on a sample of your medulloblastoma to decide which type of treatment is best.
It is for children and young people aged from 3 years old to younger than 22. We use the term ‘you’ in this summary, but if you are a parent, we are referring to your child.
Medulloblastoma is a type of brain tumour called a primitive neuro-ectodermal tumour (PNET). It is usually treated with surgery, radiotherapy and chemotherapy.
Following surgery the doctors take into account various things such as:
They then decide if you are standard or high risk. Knowing the risk group helps the doctors to come up with the best treatment plan for you. To be considered for this study you must have a standard risk medulloblastoma.
The first part of the study is called screening. Results from MRI scans you have had are looked at by the researchers. They will also look at a sample of your medulloblastoma. They do this to check if you are suitable for the treatment part of the study. From looking at your scans and checking your brain tumour sample the researchers decide if you are:
The treatment part of the study is in 2 phases. Phase 2 is for children and young people who are low biological risk and phase 3 is for children and young people who are standard biological risk
Doctors want to change existing treatment by:
They aim to improve the chances of curing your medulloblastoma and hope to reduce the severity of the long term side effects that can happen after treatment. They also want to look at how treatment affects your quality of life to see how to improve future treatments.
The following bullet points list the entry conditions for this study. Talk to your doctor or the study team if you are unsure about any of these. They will be able to advise you.
You may be able to join this study if you first have the results from your MRI scans and a sample of your medulloblastoma looked at (screening) and have been told by your doctor you have either low biological risk or standard biological risk medulloblastoma.
And if all of the following apply:
You cannot join this study if any of these apply. You
This is an international phase2/3 trial. Researchers need 60 children and young people for phase 2 and 300 for phase 3. They need about 150 children and young people in the UK to take part.
You will be in either the low risk (phase 2) or standard risk (phase 3) treatment group.
Low risk group
You have radiotherapy followed by maintenance chemotherapy. Radiotherapy starts around 4 to 6 weeks after your surgery. You have it once a day, Monday to Friday for 30 days.
Usually you would have vincristine alongside your radiotherapy but this is not being used in this study because the doctors don’t think it helps.
Your radiotherapy is carefully targeted so you have the standard dose (53.4GY) to where the tumour was and a lower dose (18GY) to the rest of your brain and spine. Doctors hope that using a lower dose of radiotherapy will help to reduce the side effects, without affecting your chances of cure.
Six weeks after you finish your radiotherapy you start your maintenance chemotherapy.
To have your chemotherapy you have a long tube that goes into your chest. You might hear it called a Hickman line or a central line. You have this put in the operating theatre. It means your doctors and nurses can take blood and give your treatment through this line.
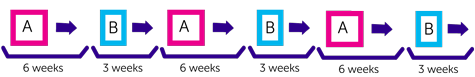
You have two different combinations of chemotherapy that your doctors call Regimen A and Regimen B. You alternate between A and B.
The following chemotherapy drugs make up Regimen A:
With Regimen A you have treatment over 2 weeks followed by 4 weeks rest.
Regimen B consists of 2 chemotherapy drugs:
You have treatment over 2 days followed by 3 weeks rest.
Each treatment period is called a cycle. You have 6 cycles in total. This takes about 27 weeks (standard maintenance chemotherapy usually lasts 48 weeks).
Standard risk group
You have radiotherapy followed by maintenance chemotherapy.
Your radiotherapy is also targeted. You have the standard dose (53.4GY) to the area where the tumour was and the standard dose (23.4GY) to the rest of the brain and spine.
Alongside the radiotherapy you might have carboplatin.
Carboplatin is already used for children and young people with high risk medulloblastoma. Now doctors want to see if it is useful for people with standard risk disease.
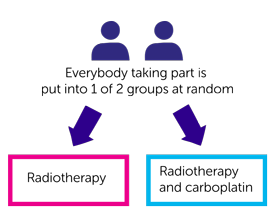
To test this, everyone in the standard risk group is randomised. This means you are put into one of two treatment groups by a computer. You have either:
Neither you nor your doctor will be able to decide which group you are in.
Your radiotherapy starts around 4 to 6 weeks after surgery. You have it once a day, Monday to Friday for 30 days. Like the low risk group, vincristine will not be given alongside radiotherapy for anyone in the standard risk group.
If you are having carboplatin, you have this every day you have radiotherapy. You have it 1 to 4 hours before radiotherapy. You have carboplatin as a drip into your central line. It takes about 1 hour to have.
Six weeks after you finish radiotherapy, you start maintenance chemotherapy.
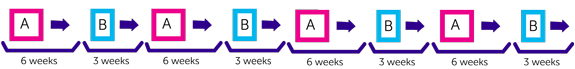
You have two different combinations of chemotherapy that your doctors call Regimen A and Regimen B. You alternate between A and B.
The following chemotherapy drugs make up Regimen A:
With Regimen A you have treatment over 2 weeks followed by 4 weeks rest.
Regimen B consists of 2 chemotherapy drugs:
You have treatment over 2 days followed by 3 weeks rest.
Each treatment period is called a cycle. You have 8 cycles in total. This takes about 36 weeks (standard maintenance chemotherapy usually lasts about 48 weeks).
Questionnaires
Everyone taking part in the trial will be asked to complete some questionnaires. You complete a questionnaire that looks at your brain (neurological and intellectual) function. You also complete other questionnaires for a Quality of life study. These have questions about:
You complete these:
You see the doctors and have some tests before starting in the trial. These include:
During both your radiotherapy and chemotherapy you have regular checks and tests. As well as repeating some of the tests above you also have:
You usually have your radiotherapy as a day patient. You will need to be in hospital for some of the time you have your maintenance chemotherapy.
Once you complete all your treatment you continue to be seen regularly by the doctors. They will check how you are and see if the treatment you had has affected your growth and development. The doctors will explain how often they’ll see you.
Radiotherapy to your head can cause short term and long term side effects.
Short term side effects include:
Long term side effects include:
We have more information on the side effects of radiotherapy to your brain.
The side effects from chemotherapy can vary according to the drugs you have but the most common general side effects include:
Your doctor will talk to you about the side effects of treatment before you start.
We have information about the side effects of:
Please note: In order to join a trial you will need to discuss it with your doctor, unless otherwise specified.
Dr Mette Jorgensen
International Society of Paediatric Oncology (SIOP)
The Brain Tumour Charity
University of Birmingham
If you have questions about the trial please contact our cancer information nurses
Freephone 0808 800 4040

"He went through six operations and was placed on a clinical trial so he could try new treatments.”